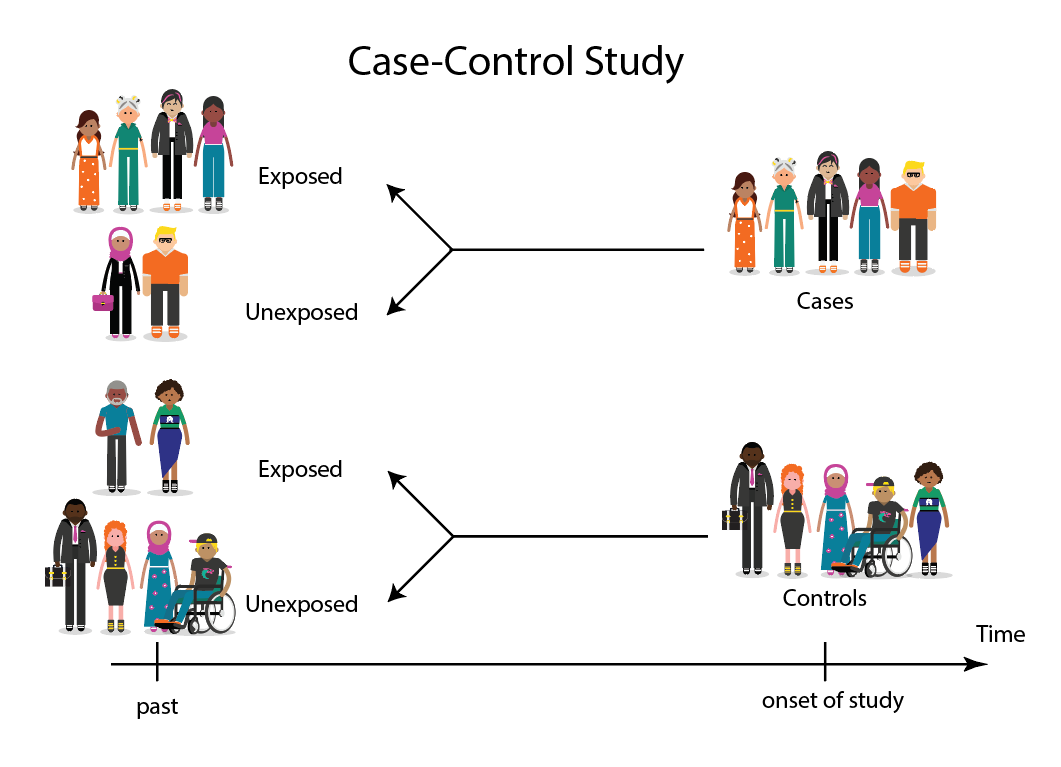
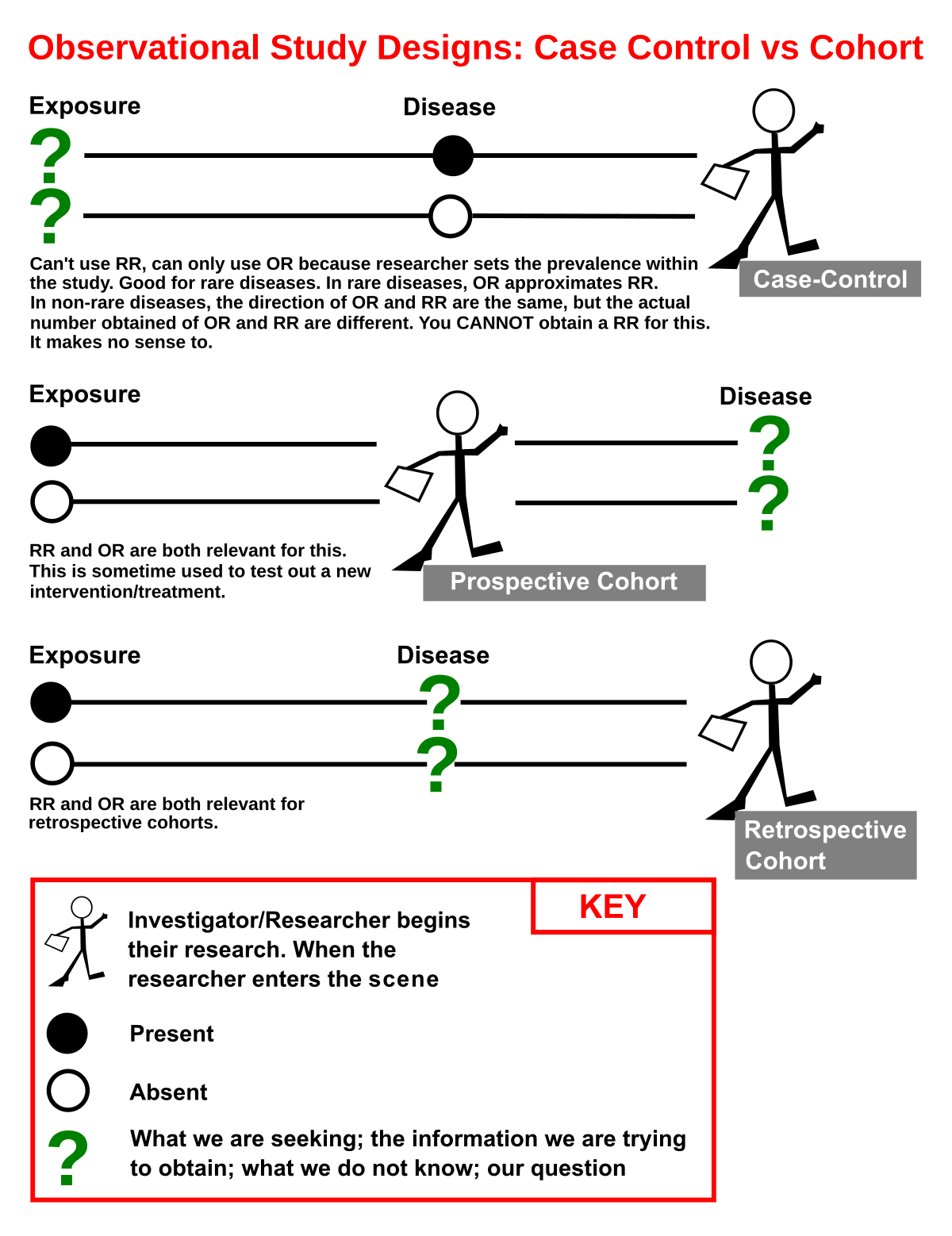
A Case-control study is a type of research that compares the health outcomes of two groups. In this type of study, one person with a disease or condition is matched with a control subject to determine whether they have been exposed to the same illness or condition. The results of this study are analyzed based on whether the treatment or control group is more effective. Case-control studies are often more effective than traditional research because they allow for a much larger sample size.
Case-control study design
When conducting a case-control study, it is important to ensure that the population being studied is representative of the disease’s overall population. This can be achieved through appropriate selection criteria. The most reliable way to ensure validity is to sample controls from the same population as the study’s cases, and to do so independently of their exposure status. In this way, the study’s sample will be representative of the disease population. Typically, case-control studies are cheaper to conduct than other studies, and they allow the study to study more rare diseases or exposures.
Case-control study sample size
A case-control study is an epidemiologic design in which a study’s population consists of both a case and a control. The cases are generally drawn from the general population, while controls are randomly selected from the same source population. The case population is usually identified by the state’s cancer registry, while controls are selected by telephone directories, voter registration lists, and tax rolls. In a case-control study, the controls are usually healthy people from the same population.
Case-control study inclusion criteria
There are several different types of case-control studies. Most case-control studies are retrospective, where subjects were enrolled at the time when they first developed the outcome under study. However, there are some distinct differences between these two types of studies. A prospective case-control study enrolls subjects based on their expected exposure to the disease or outcome of interest. The latter is typically more useful, but is more expensive and sometimes not possible. In either case, the researchers select controls from a population at risk for the disease or outcome of interest, while a retrospective study enrolls subjects after the disease has already occurred.
Case-control study results
In many studies, a biomarker is evaluated by comparing the incidence of disease in a population with that of the studied group. Case-control studies are usually conducted using a computer programme, such as Epi-Info. Case-control studies are inherently imperfect, however, because they cannot provide information on the prevalence of a disease in the general population. The authors of the study report that their analysis of the results of this study has revealed important limitations.
Case-control study odds ratio
A case-control study is a type of epidemiological research that combines two types of data: a case and a control group. Typically, a case study will have 50 subjects (cases) and 12 controls (controls). In a case-control study, the risk factor is one variable that all participants in the study will be exposed to. The odds ratio is a measure of the strength of the association between the risk factor and the outcome.