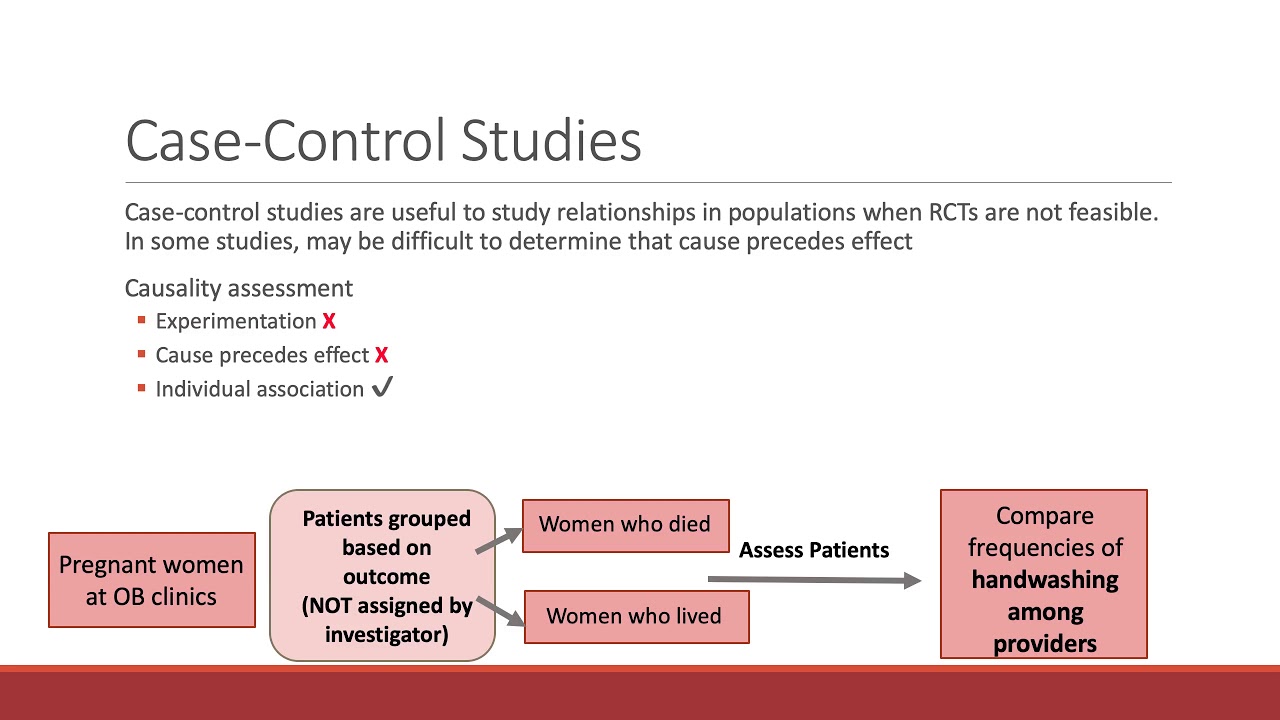
What is a case-control study? Case-control studies are observational in nature and can identify many different risk factors that may be responsible for a disease. In addition to being a cost-effective method of observation, case-control studies are also useful for identifying other possible factors that contribute to a disease. Let’s take a look at the differences between case-control studies and cohort studies and the advantages and disadvantages of each type.
Case-control studies are not a cohort study
The term ‘case-control study’ is often used incorrectly. While it’s true that many so-called case-control studies are cohort studies, the term is often not used properly. Clinicians often consider case-control studies as retrospective, and they tend to refer to patients with disease rather than healthy people. Cohort studies, on the other hand, can be both retrospective and prospective, but they are not the same.
They are observational
Case-control studies are observational studies. The results of these studies are not able to confirm that different levels of disease or risk factors are involved in the development of a certain condition. Case-control studies rely on memory of participants, who may not recall the exact circumstances surrounding the onset of the condition. The results are not representative of the general population, and may not reflect the actual cause of the condition or its consequences.
They are cost effective
Researchers can adjust a case-control study for known risk factors or uncover unknown ones. Epidemiologists, or people who study the health of populations, find case-control studies invaluable because they focus on a single risk factor. One example of a known risk factor is smoking, which researchers can control for. By following a specific set of risk factors in one group, researchers can identify more likely causes of disease and increase the effectiveness of their studies.
They can identify other possible risk factors
One advantage of case-control studies is that they can identify other possible risk factors for certain diseases. These studies start from the result of a disease and then trace the risk factor back to its exposure. The investigator has the outcome of the study in mind and uses the previously collected data to determine the risk factor for each individual. This method is particularly useful in finding other possible risk factors for certain diseases. But it is also susceptible to bias.
They are susceptible to bias
The selection bias occurs when study participants have certain characteristics in common with the cases. For example, participants with lung cancer tend to be more likely to participate than those without the condition. In this type of study, researchers can control the selection by adjusting the markers of bias. The evaluation of the selection bias can confirm that the exposure-disease associations are real. The results of a case controlled study are not valid if participants in the study are not exposed to the conditions under investigation.