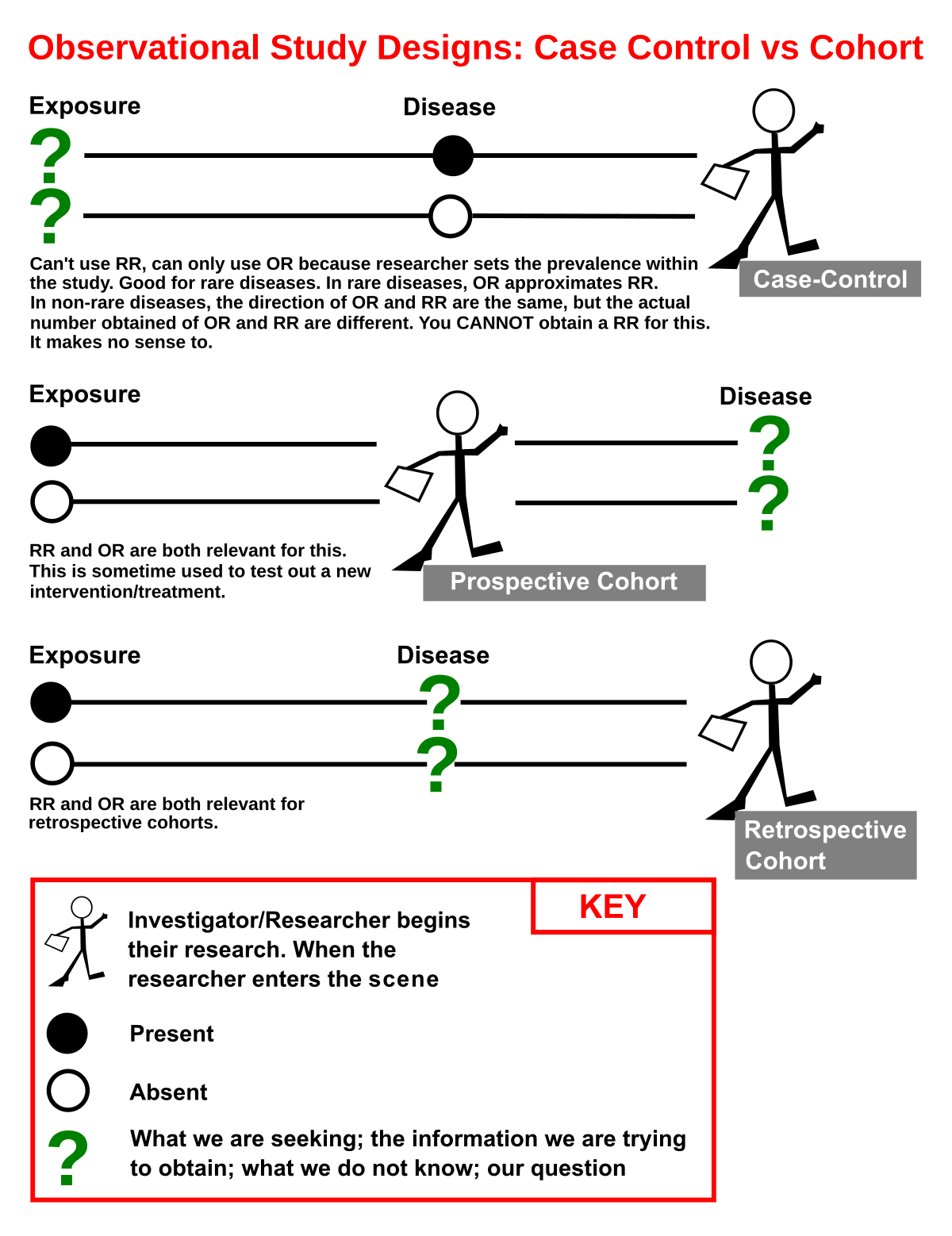
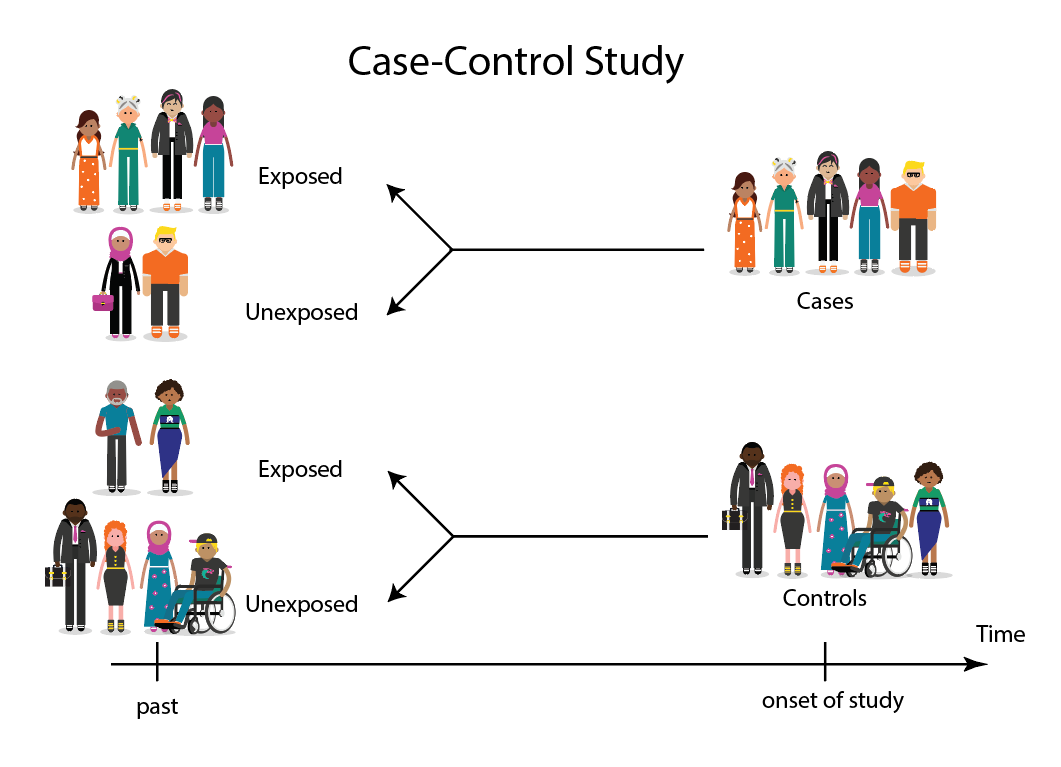
A case-control study is a type of scientific research in which cases are compared to controls in an attempt to determine whether certain factors are associated with disease. The case-control study design works backwards from the incidence of a disease to identify risk factors. Cases are more likely to have the disease than healthy controls, which is why case-control studies are the most common way to find new ways to treat or prevent disease.
Comparing cases to healthy controls
Choosing an appropriate study design is essential to minimize the potential influence of biases. A case-control study should collect information from the same populations in similar settings and from the same time period. The interviewer should not be aware of the cases’ status or specific study hypotheses, and the response rates should be comparable. This article explores three common case-control study design flaws. Let’s look at each one in more detail.
Survivor sampling
Survivor sampling in case control studies is used to identify individuals who have a specific condition or outcome. The method is similar to cumulative incidence sampling, but is applied to open cohorts, in which members may leave the study due to reasons other than the outcome of interest. Pure control sampling, on the other hand, recruits controls at the time the case is diagnosed, and therefore provides a more accurate representation of the distribution of the source population.
Matching
Case-control studies often benefit from using a matching process to compare the results of two groups of patients. Although cases and controls rarely come from the same clinic or hospital, this process ensures that the cases and controls are of the same population. By matching controls, the odds ratio for a given variable can be accurately estimated. This article will examine the pros and cons of this technique. We’ll also consider what it means for case-control studies.
Incidence rates of the disease in the source population
Case-control studies require careful selection of controls. Cases should reflect the population most at risk for developing the disease, while controls should be similar to the cases in terms of exposure to the disease. In addition, the sample population should be representative of the general population. The case-control ratio is usually 1:1, but in some cases, there may be four controls for every one case. It takes some time to select an appropriate study design. It is important to keep the disease category narrow, as this decreases the likelihood of linking specific risk factors with the incidence rate.
Methods of obtaining healthy controls
In case control studies, methods of obtaining healthy controls are crucial to the overall validity of the study. Controls should meet similar requirements to cases, such as being from the same population and at risk of developing the disease in question. They should also be chosen independently of exposures that may influence the outcome. Blind assessment, or selecting the controls before the cases are known, is the optimal method for obtaining healthy controls.